
Temperature-Controlled Storage.
Storage regulations are tightening, can your storage strategy keep up?
Staying compliant across multi-national operations can be challenging with product traceability, documentation and even audit readiness to consider.
In strict accordance with MHRA, cGMP and GDP regulations, we provide a range of pharmaceutical warehousing options including temperature controlled, refrigerated and ambient monitored storage. We offer 6,000+ pallet storage locations and temperature controlled warehousing with complete regulatory support, including onsite QP, serialisation and more…
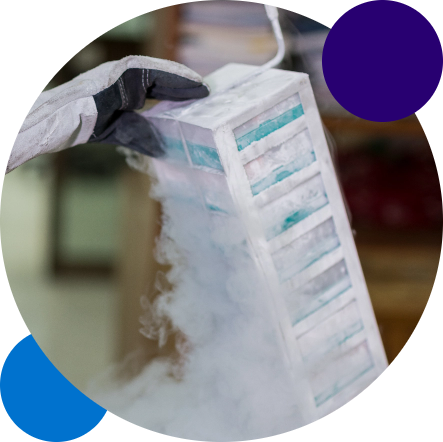
Overview:
We use a fully computerised stock control system for efficient and accurate stock management and movement of your temperature-controlled products.
✅ Guaranteed monitored temperature deliveries
✅ Live GPS location mapping
✅ Fully scalable delivery packaging options
✅ Multi-day temperature compliance
✅ Full contingency delivery programmes
✅ Cleanrooms for qualified kitting for forward sterile manufacture
✅ Extensive guaranteed warehousing to meet small to large scale demand
✅ Third-party laboratory services
✅ Ambient, 2-8, -20, -80 degree (and Liquid Nitrogen temperature-controlled storage coming soon)
✅ 24-hour continuous temperature monitoring with real-time visibility
✅ Up to same day stock call-off and delivery.
Temperature-controlled storage options can complement our contract packing services or act as a standalone service for clients looking for 3PL support.
Accreditations & Licences.




